Monday, May 19, 2008
Student Union Building, Upper (Queensborough Community College)
332
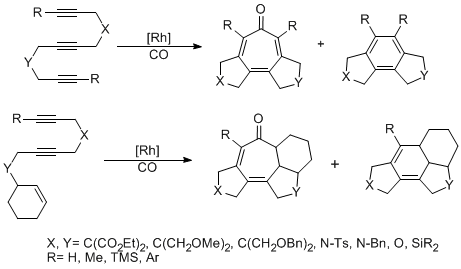
Formation of Novel Fused-Ring System by Rhodium-Catalyzed Cycloaddition Reactions
We have investigated the Rh-catalyzed [2+2+2+1] and [2+2+2] clycloaddition of triynes and cyclohexenyldiynes. The cycloaddition of triynes gave 5-7-5 and 5-6-5 tricyclics and the cycloaddition of cyclohexenyldiynes gave 5-7-5-6 and 5-6-5-6 tetracyclics. These ring systems are of interest due to their presence in natural products and biologically relevant compounds. The Rh-catalyzed [2+2+2+1] tricyclization of triynes took place to give tropones, which should provide a potentially useful method for the synthesis of interesting troponoids. On the other hand, the Rh-catalyzed [2+2+2] cycloaddition of triynes gave annulated benzenes, an important class of synthetic intermediates in natural product synthesis.