Monday, May 19, 2008 - 2:35 PM
Medical Arts Building, Rm M-142 (Queensborough Community College)
238
Organic Rigid-Rods with Para- and Meta- Conjugated Bridges for Semiconductor Nanoparticles Sensitization
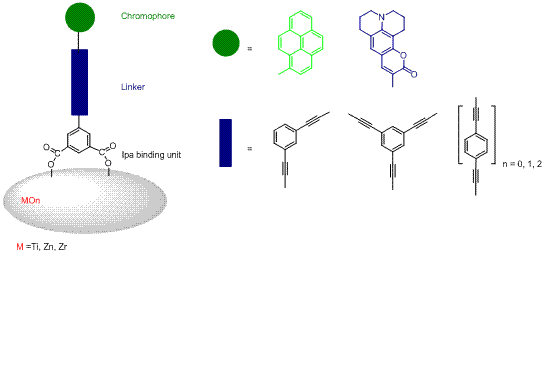
Rigid-rods with organic chromophores (Pyrene and Coumarin 6H) having a oligophenylenethynylene (OPE) rigid linker, linear or branched, were synthesized and bound to MOn (TiO2, ZrO2, and ZnO) nanoparticle thin films through isophthalic (Ipa) groups anchoring groups (see Figure). The influence of the linker's length and branching on the photophysical properties of the compounds has been studied in solution and on MOn surface. All synthesized rigid-rods showed high quantum yields and high extinction coefficients when compared to the parent compounds. Photophysical, photoelectrochemical, and electrochemical studies been complemented with quantum mechanical calculations. The nature of the binding of the organic sensitizers to MOn was characterized by FTIR-ATR and found to be dependent on the pH pretreatment of the semiconductor surface. The aggregation phenomena of the organic dyes were studied using ZrO2 (an insulator, bandgap ~ 5 eV) films. Pyrene rigid-rods formed excimers on the metal oxide surface whereas coumarin rigid-rods produced non-emissive aggregates. Photoelectrochemical studies of the pyrene rigid-rods in regenerative solar cells showed near quantitative conversion of absorbed photons into electricity. Work in progress will be discussed.