Friday, 18 May 2007
3rd Floor Hall (Pfahler Hall)
474
Synthesis of sulfur-substituted quinones from the oxidation of 5-methyl-4-mercapto-catechol and 5-methyl-3,4-dimercapto-catechol
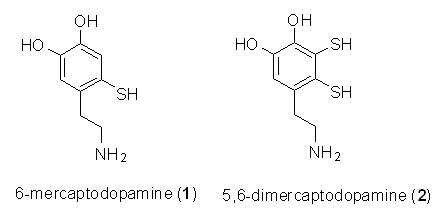
Quinones derived from 6-mercaptodopamine (1) and 5,6-dimercaptodopamine (2) may serve as precursors for sulfur-containing natural products, such as thianthrenes and tetrathiocins extracted from marine invertebrates. Thus, we undertook an experimental effort to generate possible quinones from a model system, which used 4-mercapto-5-methyl catechol (3) and 3,4-dimercapto-5-methyl catechol (4) as reagents. The formation of quinones and dimers from the oxidation of 3 and 4 are assessed by NMR, HPLC, and mass spectrometry. The experimental data will also be compared with our DFT computations.
Back to Poster Session V
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)