Friday, 18 May 2007
3rd Floor Hall (Pfahler Hall)
464
Aziridine metalation, silylation and ring opening: a general synthetic approach to α-aminosilanes and silanediol-based protease inhibitors
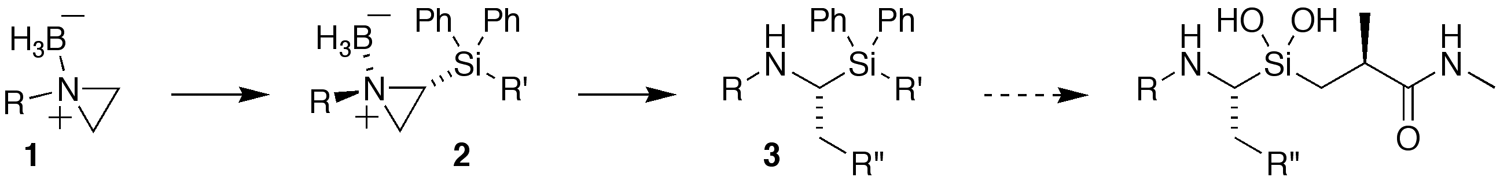
Vedejs' lithiation/silylation of aziridine – borane complexes 1 can be performed enantioselectively. The chiral aziridinium 2 can then be opened with cuprates to yield the α-amino silanes 3. Aziridine-borane complexes 1 are stable and can be purified by standard silica gel chromatography. Follow ring opening, however, the borane is readily removed by simple exposure to methanol to give 3. This method provides a general approach to optically active α-amino silanes, including silanediol-based protease inhibitors.
Back to Poster Session V
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)