Friday, 18 May 2007
3rd Floor Hall (Pfahler Hall)
463
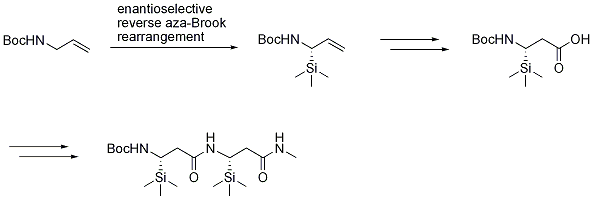
Application of reverse aza-Brook rearrangement in the asymmetric synthesis of β-silyl β-amino acids and peptides
β-Silyl β-amino acids and derivatives have proven utility as building blocks for molecules with applications in pharmaceutical and material science. Silicon-containing β-amino acids are virtually unknown, but are expected to exhibit different properties than the carbon analogues, such as lipophilicity. Reverse aza-Brook rearrangement reaction was efficiently applied in the synthesis of β-silyl β-amino acids. β-Silyl dipeptide has been made from the corresponding β-silyl β-amino acid.
Back to Poster Session V
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)