Friday, 18 May 2007
3rd Floor Hall (Pfahler Hall)
461
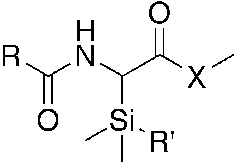
Synthesis and stability of alpha-silyl amino acids and their derivatives
A series of alpha-silyl amino acids and their polypeptide derivatives have been studied to determine their stability and potential for applications in unnatural polypeptides and pharmaceuticals. These amino acids were prepared from furfurylamine, via reverse aza-Brook rearrangement followed by ozonolysis. The C-Si bond stability toward hydrolysis and methanolysis was evaluated as a function of the substituents on silicon, nitrogen and the carbonyl group, revealing an interesting interplay of steric and electronic effects.
Back to Poster Session V
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)